
Describe the formation of single covalent bonds in H 2, C l 2, H 2O, CH 4, NH 3 and HC l as the sharing of pairs of electrons leading to the noble gas configuration.Ī covalent bond is formed when atoms share electrons.Ī hydrogen atom has only one electron in the first shell.Sometimes, however, these steps do not work. This is a good Lewis electron dot diagram for BF 4 −. The B atom has eight electrons around it, as does each F atom. There are no additional electrons to add to the central atom.Ħ. Put remaining electrons, if any, around the central atom. This uses up 24 more electrons, leaving 24 − 24 = 0 electrons left.ĥ. Complete the octets around the surrounding atoms (except for H). This uses up eight electrons, so we have 32 − 8 = 24 electrons left.Ĥ. Put a pair of electrons between the central atom and each surrounding atom. Write the central atom surrounded by surrounding atoms.ģ. B has 3, each F has 7, and there is one extra electron: 3 + 7 + 7 + 7 + 7 + 1 = 32.Ģ. There is a negative sign on the species, so we have an extra electron to consider.ġ. The B atom is the central atom, and the F atoms are the surrounding atoms. Let us try these steps to determine the electron dot diagram for BF 4 −.
#Carbon dioxide formula ionic or covalent full#
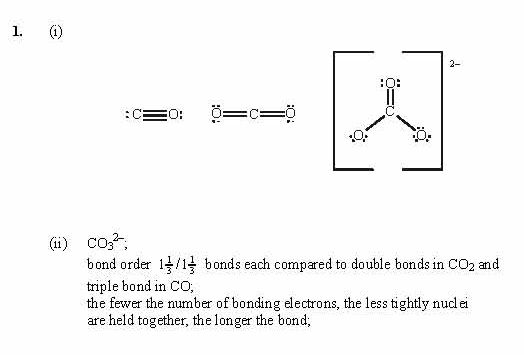
Check that every atom has a full valence shell.Put remaining electrons, if any, around the central atom.Complete the octets around the surrounding atoms (except for H).Put a pair of electrons between the central atom and each surrounding atom.Write the central atom and surround it with the surrounding atoms.Add extra if the species has negative charges and remove some for every positive charge on the species. Count the total number of valence electrons.After the central and surrounding atoms have been identified, follow these steps: The central atom is usually written first in the formula of the compound (H 2O is the notable exception). The central atom is the atom in the center of the molecule, while the surrounding atoms are the atoms making bonds to the central atom. First, you must identify the central atom and the surrounding atoms. There is a simple set of steps for determining the Lewis electron dot diagram of a simple molecule. Note that the H atom has a full valence shell with two electrons, while the F atom has a complete octet of electrons. The two atoms can share their unpaired electrons to make a covalent bond: Each atom starts out with an odd number of electrons in its valence shell: Each F atom has one bonding pair and three lone pairs of electrons.Ĭovalent bonds can be made between different elements as well. Each F atom has three other pairs of electrons that do not participate in the bonding they are called lone pair electrons. The bonding electron pair makes the covalent bond. There are two different types of electrons in the fluorine diatomic molecule. We can also write this using a dash to represent the shared electron pair: Note that each F atom has a complete octet around it now: These two atoms can do the same thing that the H atoms did they share their unpaired electrons to make a covalent bond. F atoms have seven electrons in their valence shell: (This explains why hydrogen is one of the diatomic elements.) For simplicity's sake, it is not unusual to represent the covalent bond with a dash, instead of with two dots:īecause two atoms are sharing one pair of electrons, this covalent bond is called a single bond. We can use circles to show that each H atom has two electrons around the nucleus, completely filling each atom's valence shell:īecause each H atom has a filled valence shell, this bond is stable, and we have made a diatomic hydrogen molecule. The two H atoms can share their electrons:


 0 kommentar(er)
0 kommentar(er)
